
Pharmaceutical Industry
Strict standards and guidelines apply in the pharmaceutical industry. Regulatory authorities, such as the US Food and Drug Administration (FDA) and the European Medicines Agency (EMA), provide detailed procedures and documentation. Using Statistica and the know-how of StatSoft, we support you in setting up solutions for efficient data analysis in validated system environments.
Validated Reporting (APR/PQR)
Generating Annual Product Reviews (APR in the USA) or Product Quality Reviews (PQR in Europe) is a very time-consuming and cost-intensive process. Using Statistica, you can automate the APR and PQR creation process to the greatest possible extent.
Shelf-Life Estimation
Companies in the pharmaceutical industry use statistical methods to calculate the runtime of a product or the repeat testing period of an active ingredient during long-term storage. This is because there are strict requirements from the European approval authorities. The Statistica add-in Shelf-Life Estimation enables you to perform automated calculations according to Q1E specifications.
Trending
In order to consistently monitor processes and gain an improved understanding of them, you can use Statistica in an optimal way. Statistica naturally complies with the strict GxP standards.
Transformation of data management and analytics
How can you successfully transform your data management and analytics?
The cooperation of StatSoft EUROPE and the international Pharma company CSL Behring shows, how considerable added value can be generated.

Request the case study as download now.
Your contact data
Pharmaceutical and laboratory solutions
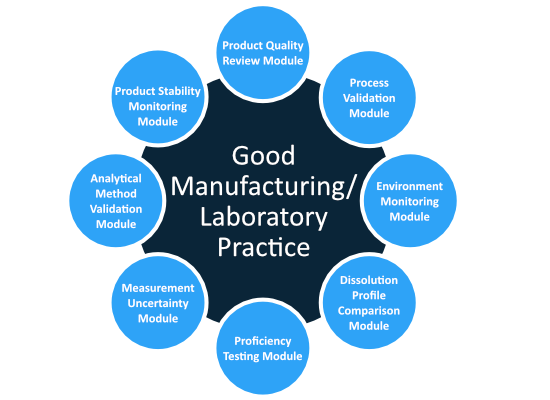
All solutions enable the standardization of statistical data analysis and analysis as well as the automatic generation of reports in the form of tables and / or graphics and thus contribute to process optimization.
Our applications include the trend towards product stability, monitoring of the production environment, process validation and ongoing review, regular product reviews, solution profiling, and assessment and validation of uncertainties of analytical methods.

 Testing
Testing Contact
Contact Search
Search